Projects
Singlet Fission • Heteroacenes and FETs • Catalysis and Mechanistic Studies • Conjugated Oligomers • Foldamers and Nanographenes • Photodetectors • PAH Dyads
Pentacene-Based PAH Dyads and Polarized Pentacenes |
One of the benefits of developing organic materials is the ability to systematically vary substituents attached to the chromophore to fine-tune properties such as the HOMO and LUMO energies, which in turn influence the UV-vis absorption characteristics and the redox properties. These structural variations facilitate the elucidation of structure-property relationships which provide guidance for the design of materials with targeted properties. Since non-covalent interactions play an important role in dictating the supramolecular arrangement of these small molecules, varying the substituents also allows for tuning of the morphology, in films and crystals, which in turn influences the device performance. Thus, the appropriate choice of substituents can lead to significant improvements in device performance.
Figure 1. Electronically polarized pentacenes featuring electron donating and withdrawing groups.
The development of synthetic routes to unsymmetrically substituted pentacenes has allowed for the development of electronically polarized pentcenes,[1] via the incorporation of electron donating or withdrawing groups, as well as pentacene-based polycyclic aromatic hydrocarbon dyads.[2] These structural modifications provide a way to tune various optical, electrochemical and solid-state packing properties in order to achieve materials with optimized properties for various particular device applications.[3]
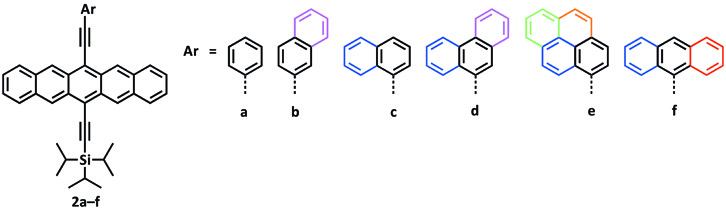
Figure 2. Pentacene-Based Polycyclic Aromatic Hydrocarbon (PAH) Dyads.
The development of a homologous series of pentacene–PAH dyads (2a—f, Figure 2) has been realized [2] in which the absorption is tuned to incorporate additional absorption bands in the region from 350—500 nm where pentacene derivatives are normally quite transparent.[3] The design of these targets is based on three key factors. First, a PAH should be attached to the pentacene through an ethynyl linker at the 13-position to provide extended conjugation and enhance absorption in the 300—500 nm range. Second, the increased π-surface provided by the PAH moiety should offer improved π-stacking interactions in the solid-state. Finally, a triisopropylsilylethynyl group should be appended to the 6-position to maintain the solubility of the product.
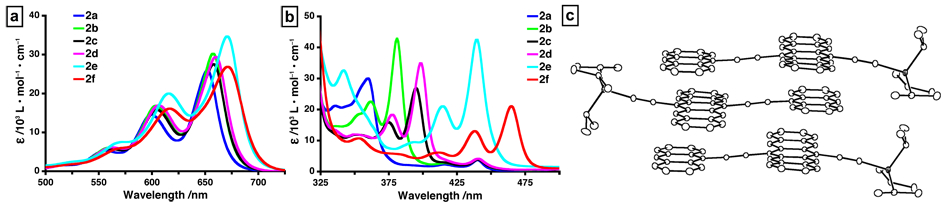
Figure 3. Solution-state UV-vis absorption spectra for dyads 2a—f in CH2Cl2. (a) Low-energy region (500—725 nm) and (b) mid-energy region (325—500 nm) of spectra. (c) X-ray crystallographic structure of 2f illustrating the π-stacking in the solid-state.
[1] D. Lehnherr, R. McDonald, R. R. Tykwinski, Org. Lett. 2008, 10, 4163—4166. [PDF]
[2] D. Lehnherr, A. H. Murray, R. McDonald, M. J. Ferguson, R. R. Tykwinski, Chem. Eur. J. 2009, 15, 12580—12584. [PDF]
[3] D. Lehnherr, R. R. Tykwinski, Materials 2010, 3, 2772—2800. [PDF]
[4] D. Lehnherr, M. Adam, A. H. Murray, R. McDonald. F. Hampel, R. R. Tykwinski, Can. J. Chem. 2017, 95, 302—314. [PDF]