Projects
Singlet Fission • Heteroacenes and FETs • Catalysis and Mechanistic Studies • Conjugated Oligomers • Foldamers and Nanographenes • Photodetectors • PAH Dyads
Conjugated Pentacene Oligomers |
The systematic study of conjugated pentacene oligomers with different linkers, substituents, and number of repeat units has allowed for the elucidation of structure-property relationships. Varying these parameters allows for fine-tuning of properties such as absorption characteristics, redox properties, solid-state packing and HOMO-LUMO gap. Two synthetic methods are illustrated below which facilitate access to these highly conjugated systems. Scheme 1 illustrates both a stepwise and a one-pot route that allow for the desymmetrization of the pentacene core via sequential addition of different acetylides (1 then 2) to pentacenequinone (3) to afford unsymmetrical pentacenes (4) after the reductive aromatization of diol 5. Expanding upon the one-pot route, the second acetylide added can be a di-acetylide (6), this now results in dimerization of 7b to a tetra-ol (8). This one-pot route rapidly assembles five fragments in one step, and after a subsequent reductive aromatization of tetraol 8, conjugated pentacene dimers (9) can be obtained.
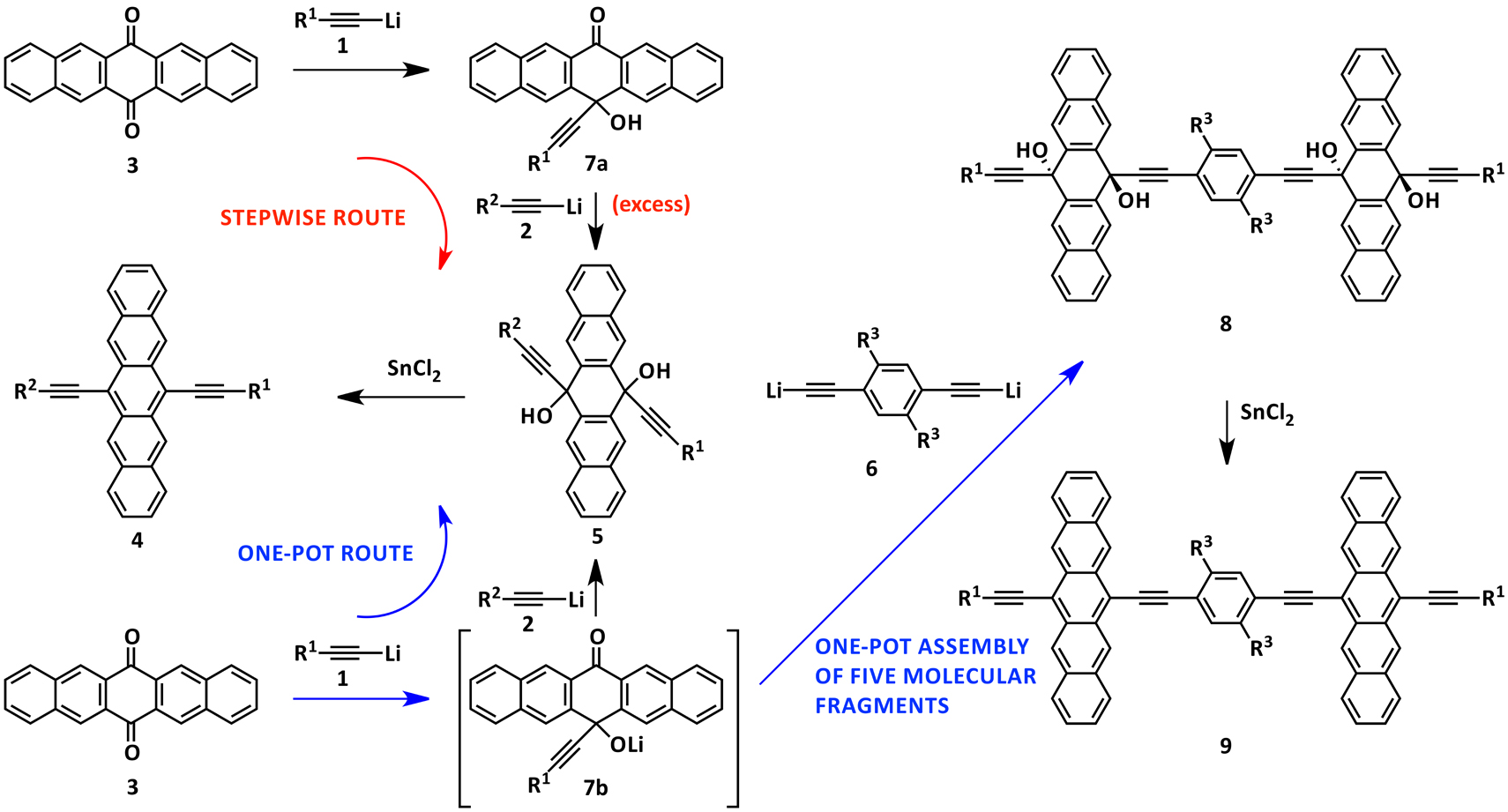
Scheme 1. Synthetic routes developed to desymmetrize the pentacene core which allows for the formation of conjugated dimers.
In order to access longer oligomers (trimers and higher), new methodology was required. The method described in Scheme 1 is only amenable to the synthesis of dimers, thus an operationally simple and modular method to conjugated pentacenes was developed. The formation of unsymmetrical pentacene diols protected as methyl ethers allowed for the use of metal catalyzed homo- and cross-coupling methods based on Cu- and Pd-chemistry. Dihydropentacene intermediates (10a—c) with a variety of solubilizing groups can be obtained in gram scale quantities and could be stored as solids until their required use in homo- and cross-coupling reactions for the formation of pentacene oligomers (12—14, Scheme 2).
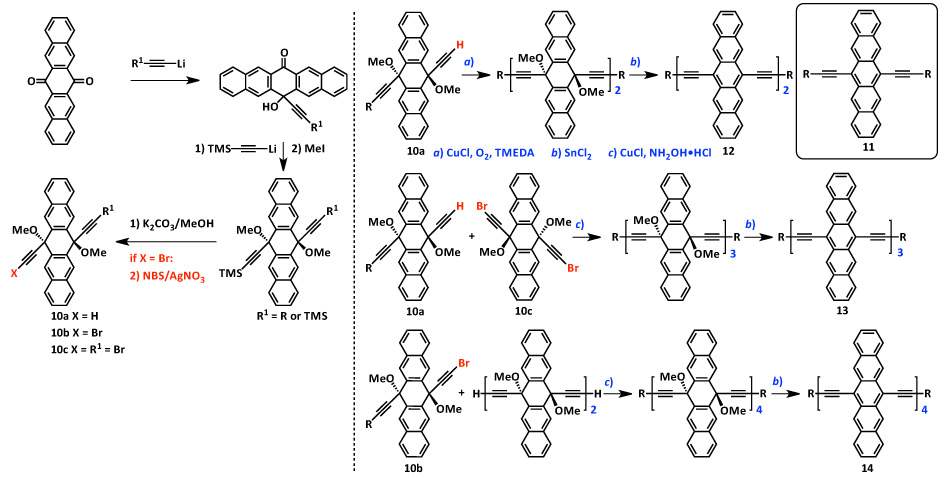
Scheme 2. Modular assembly of a homologous series of conjugated pentacene oligomers (11—14).
The synthesis of a homologous series of diethynylpentacene oligomers linked was studied by UV-vis and electrochemical methods. As the oligomer length is increased (11—14), a concurrent red shift in the UV–vis absorption lambdamax values is observed (Figure 1a), indicative of increased conjugation with the HOMO–LUMO gap dropping from 1.86 eV (11) to 1.57 eV (12) to ca. 1.38 eV for both 13 and 14 (Figure 1). It can be noted that trimer 13 and tetramer 14 possess a remarkably low band gap for a material composed of only hydrocarbon chromophores.
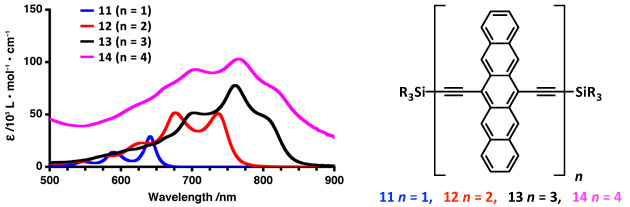
Figure 1. UV-vis absorption spectra of pentacene oligomers 11—14 as a function of the number of repeat units illustrating near-saturation of the absoption characteristics via nearing the effective conjugation length with tetramer 14.
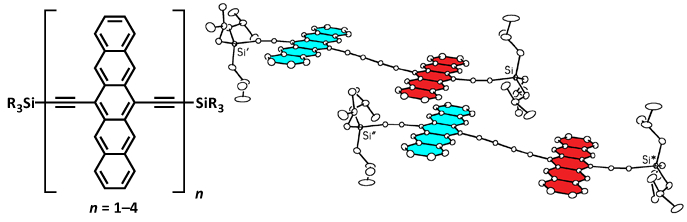
X-ray crystallographic analysis confirms the ability of these oligomers to π-stack. For example, the solid-state geometry of 12 reveals that the two pentacene moieties of 12 adopt a pseudo-coplanar arrangement in which the packing features a 2-D slipped stacked arrangement (Figures 2) This 2-D slipped stack arrangement, together with the ability of the butadiynyl linker to facilitate electronic communication, provides 3-D electronic communication in the solid-state, which could greatly simplify device formation, something that had yet to be achieved with any functionalized pentacene!
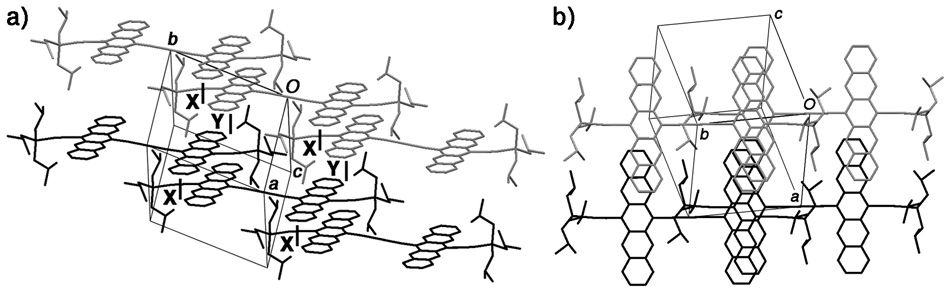
Figure 2. X-ray crystallographic structure of dimer 12 illustrating (a) the interaction of neighboring pentacene dimers in the staircase along the crystallographic b axis (labeled X) and of two adjacent staircases along the crystallographic a axis (labeled Y). (b) Effective overlap of pentacene moieties as viewed from above.
[1] D. Lehnherr, A. H. Murray, R. McDonald, R. R. Tykwinski, Angew. Chem. Int. Ed. 2010, 49, 6190—6194. [PDF]
[2] D. Lehnherr, J. Gao, F. A. Hegmann, R. R. Tykwinski, Org. Lett. 2008, 10, 4779—4782. [PDF]
[3] D. Lehnherr, R. R. Tykwinski, Aust. J. Chem. 2011, 64, 919—929. [PDF]