Projects
Singlet Fission • Heteroacenes and FETs • Catalysis and Mechanistic Studies • Conjugated Oligomers • Foldamers and Nanographenes • Photodetectors • PAH Dyads
Nanographenes, ortho-arylenes, and [N]Phenylenes via Benzannulation of Acetylenes |
Leveraging transition metal-catalyzed benzannulation of acetylene derivatives (haloalkynes, silylhaloalkynes and arylacetylenes) enanables the rapid formation of elaborate naphthalene derivatives. Through the design of the acetylene precursor, highly substituted naphthalenes, or oligomers and polymers thereof, can be rapidly assembled which are precursors to larger aromatic structures (via oxidative C-C bond fromation). This strategy enables access to nanographenes, [N]phenylenes, graphene nano-ribbons (GNRs), as well as heteroaromatic structures (Figure 1), all of which are potentially useful for electronic applications (e.g. transistors, solar cells, display technologies, energy storage).
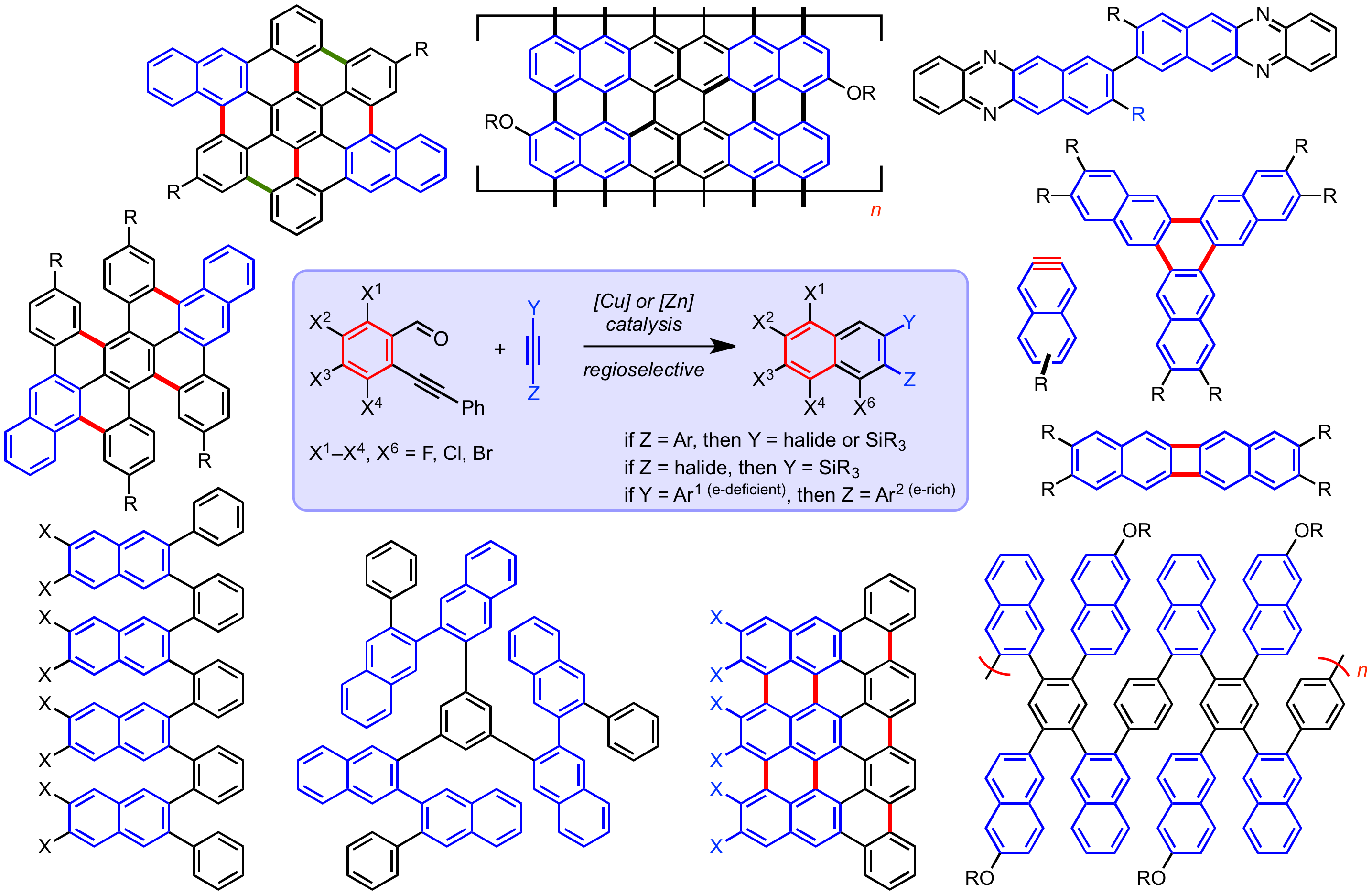
Figure 1. Illustration ortho-Arylene, [N]Phenylenes, Heteroarenes, Nanographenes, and Graphene Nano-Ribbons attainable by leveraging Cu- and Zn-catalyzed benzannulation of arylacetylenes, haloalkynes, and silylhaloalkynes.
Complimentary to the Cu-catalyzed benzannulation of haloalkynes, a Zn-catalyzed benzannulation of silylhaloalkynes affords silylhalonaphthalenes; both methods being highly regioselective (Figure 2). We demonstrated that ortho-silylhalonaphthalenes serve as naphthyne precursors amenable to cycloaddition, polymerization and oligermization to [N]phenylenes via either dimerization or trimerization processes.
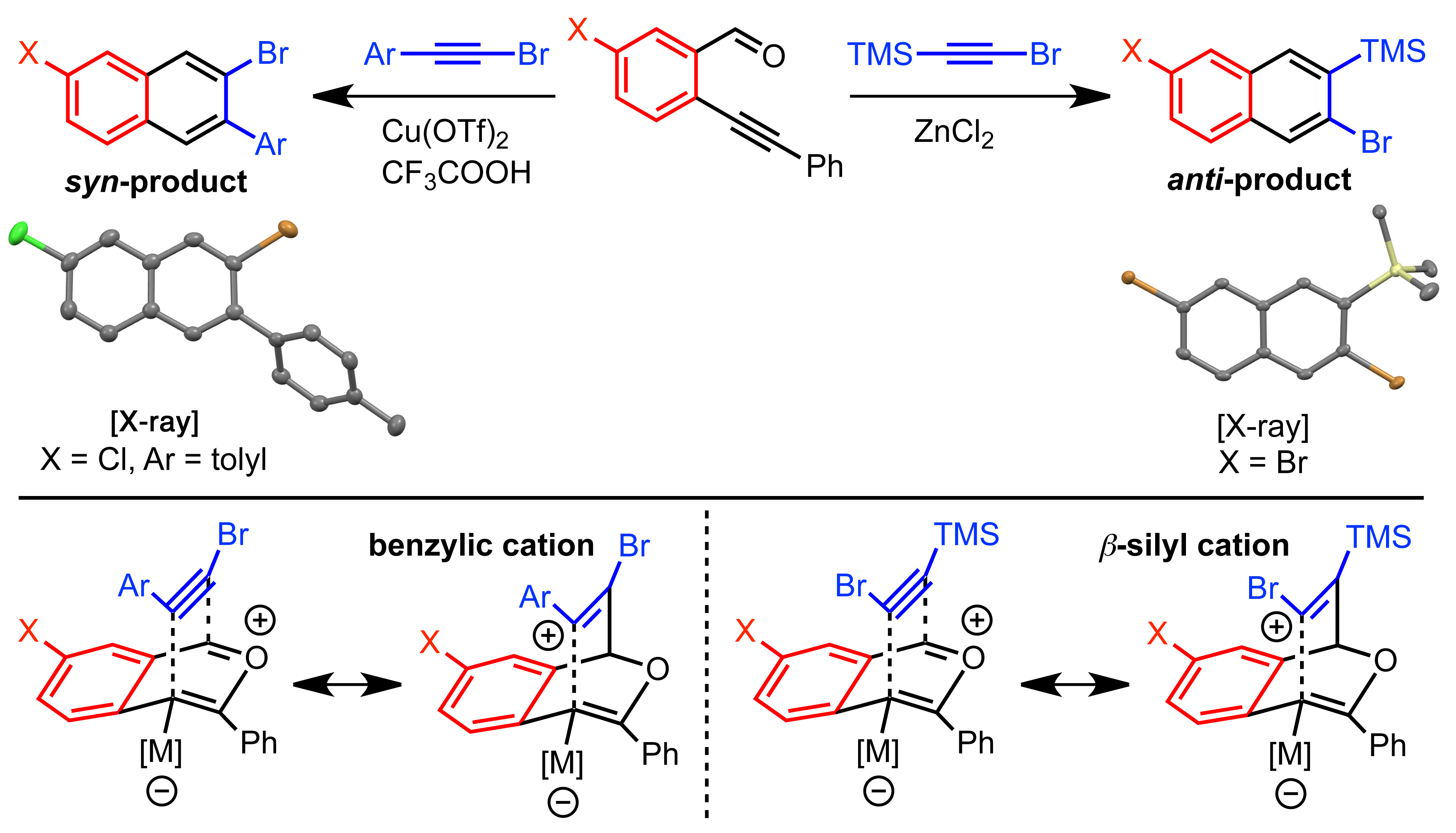
Figure 2. Illustration of regioselective models for the Cu- and Zn-catalyzed benzannulation of haloalkynes and silylhaloalkynes.
[1] S. J. Hein, D. Lehnherr, H. Arslan, F. J. Uribe-Romo, W. R. Dichtel, Acc. Chem. Res. 2017, 50, 2776—2788. [PDF].
[2] D. Lehnherr, J. M. Alzola, E. B. Lobkovsky, W. R. Dichtel, Chem. Eur. J. 2015, 21, 18122—18127. [PDF].
[3] D. Lehnherr, C. Chen, Z. Pedramrazi, C. R. DeBlase, J. M. Alzola, I. Keresztes, E. B. Lobkovsky, M. F. Crommie,
W. R. Dichtel, Chem. Sci. 2016, 7, 6357—6364. [PDF].
[4] S. J. Hein, D. Lehnherr, W. R. Dichtel, Chem. Sci. 2017, 8, 5675—5681. [PDF].
[5] S. J. Hein, D. Lehnherr, W. R. Dichtel, J. Org. Chem. 2017, 82, 2004—2010. [PDF].
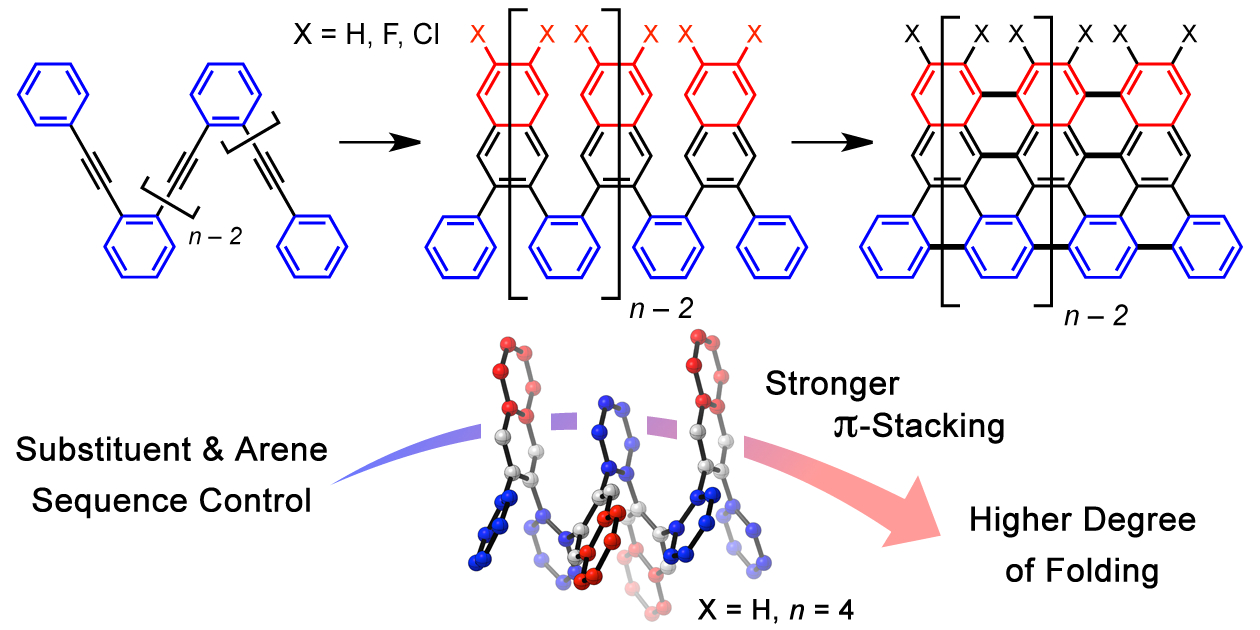
ortho-Arylene Foldamers |
Oligomers and polymers based on linking arenes are ubiquitous in materials chemistry. From a structural standpoint, oligo- and polyphenylenes are among the most simple motifs, while poly(para-phenylene)s and poly(meta-phenylene)s have been studied extensively, oligomers and polymers based on ortho-arylenes are much less explored because they are more difficult to access. We developed a Cu-catalyzed benzannulation method to transforms ortho(arylene ethynylene) oligomers into ortho-arylenes.
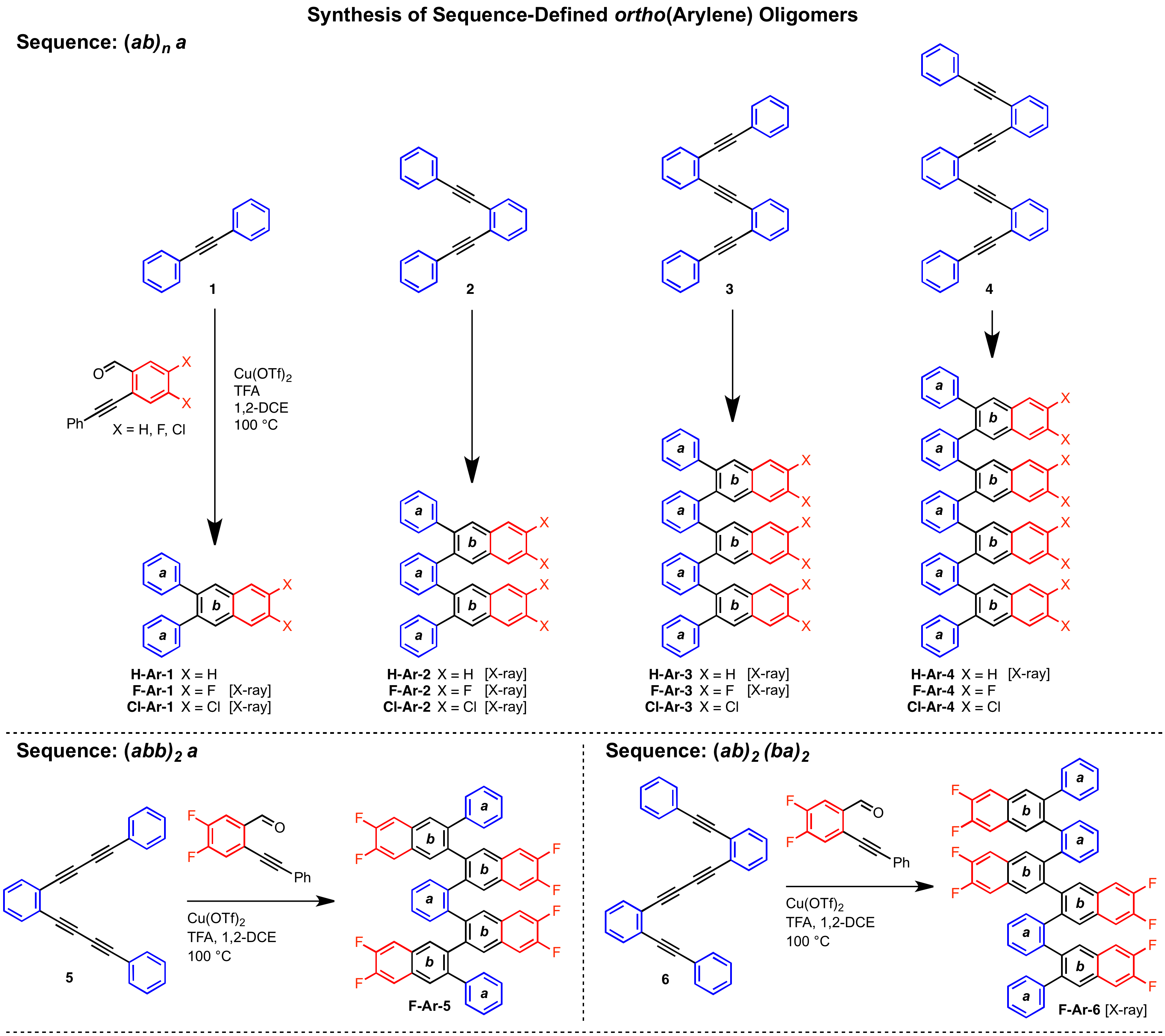
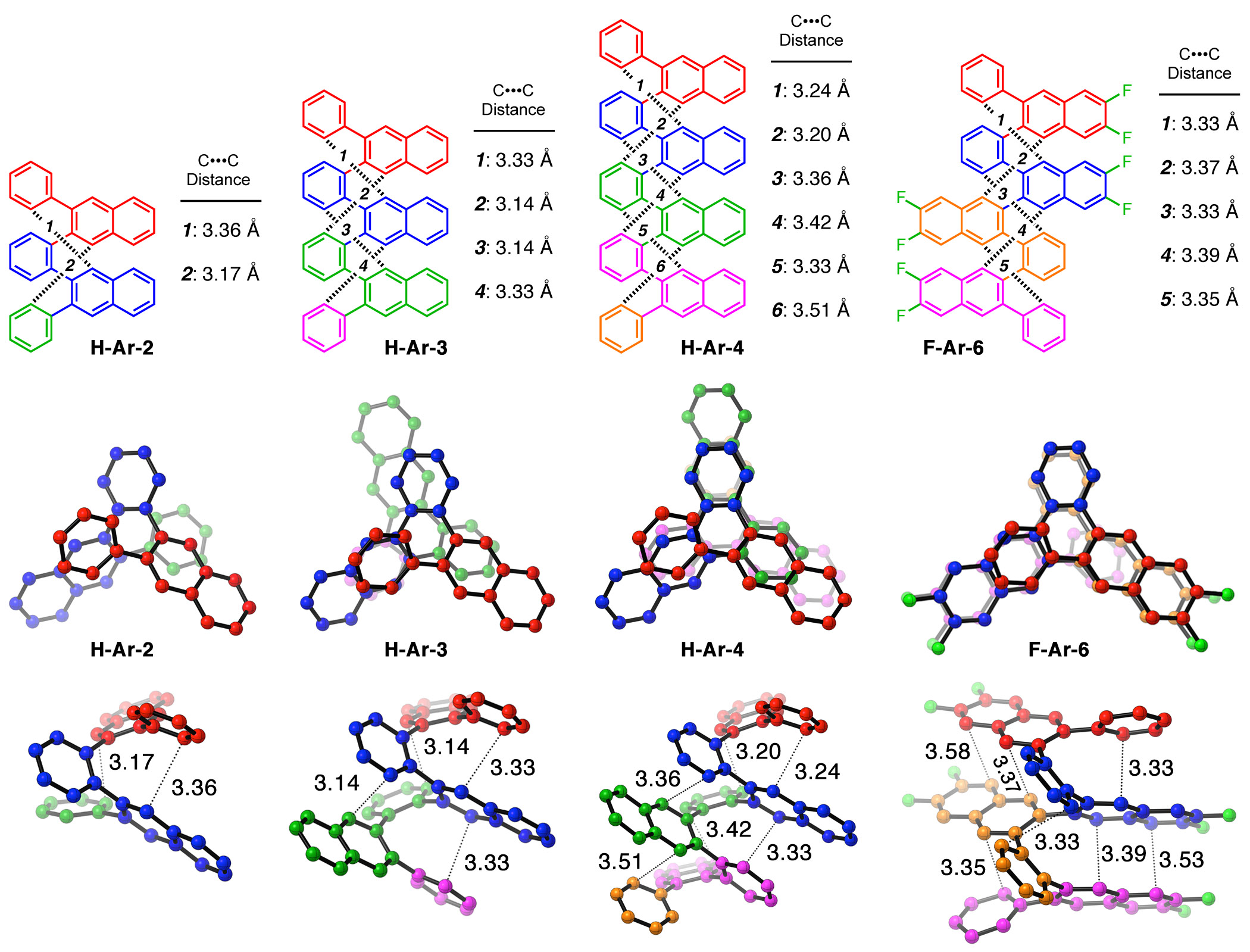
These derivatives form helical folded structures in the solid-state and in solution, as demonstrated by X-ray crystallography and solution-state NMR analysis. DFT calculations of misfolded conformations were correlated with variable-temperature 1H and EXSY NMR spectroscopy to reveal that folding is cooperative and more favorable in halide-substituted naphthalenes. Helical ortho-arylene foldamers with specific aromatic sequences organize functional π-electron systems into arrangements ideal for ambipolar charge transport and show utility for the surface-mediated synthesis of structurally defined graphene nanoribbons.
[1] D. Lehnherr, C. Chen, Z. Pedramrazi, C. R. DeBlase, J. M. Alzola, I. Keresztes, E. B. Lobkovsky, M. F. Crommie,
W. R. Dichtel, Chem. Sci. 2016, 7, 6357—6364. [PDF].
Nanographenes from ortho-Arylene Precursors |
The ability to access o-arylenes and introduce functionality, such as halides, on the arenes in the final step opens up new opportunities. For example, polymeric derivatives of these foldamers might serve as precursors to graphene nanoribbons, in which this bottom-up approach enables the control of width, length, and edge structure (e.g., hydrogen vs halogen edge termination). As a proof of principle, the conversion of foldamer H-Ar-3 to polycyclic aromatic hydrocarbon H-8 was demonstrated via an on surface-mediated oxidation process.
Evaporation of H-Ar-3 in ultra high vacuum enabled its deposition onto Au(111). The STM image in Figure 2A illustrates a surface coverage of 0.8 monolayers of H-Ar-3 on Au(111) obtained after deposition of the H-Ar-3 molecules. The molecules are seen to form ordered crystalline domains approaching ca. 20 nm (a diagonal grain boundary separating two such domains is visible in Fig. 2A). At lower surface coverage, H-Ar-3 selectively attaches to step edges of Au(111) (Fig. 2B). A close up of several molecules (Fig. 2C) clearly shows the non-planarity of H-Ar-3, which exhibits an apparent height of 3–5 Å depending on molecular conformation. Upon annealing the H-Ar-3/Au(111) to 286 °C, a change in the shape of the molecules is observed consistent with conversion to H-8. Fig. 2D shows the dominant product (60%): a planar, trapezoidal molecule with measured height of 1.9 ± 0.1 Å. The dimensions of this structure (1.9 ± 0.2 nm x 1.3 ± 0.2 nm) are consistent with DFT predicted dimensions of H-8 (Fig. 2E).
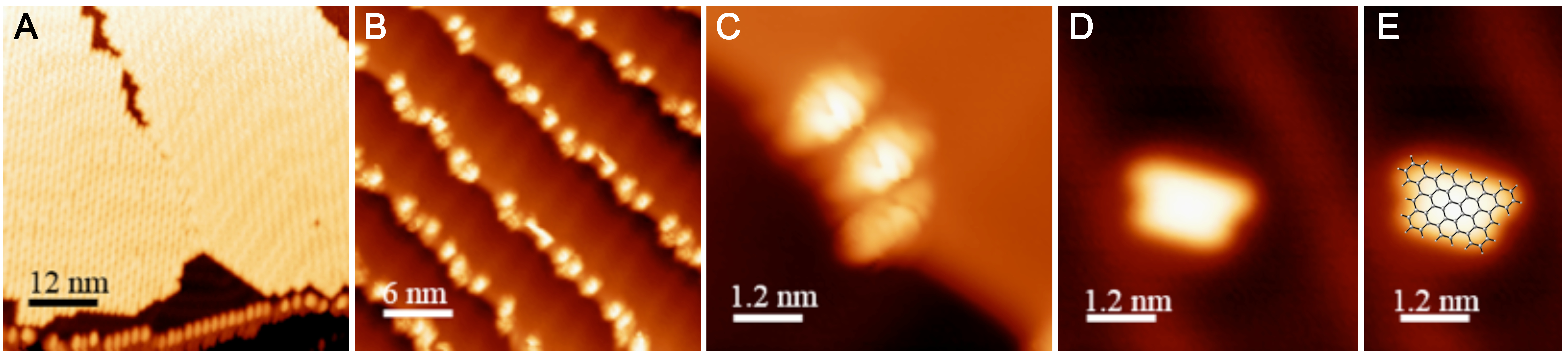
Figure 2. STM images of H-Ar-3 and H-8 molecules on Au(111). (A) 0.8 monolayer coverage of H-Ar-3 as deposited shows well-ordered crystalline domains. (B) Lower surface coverage of H-Ar-3 as deposited shows molecular adhesion to step edges of Au(111) crystal. (C) Close-up of several molecules of H-Ar-3 before annealing reveals non-planarity. (D) Individual nanographene H-8 obtained after annealing to 286 °C. (E) DFT optimized structure of H-8 overlaid on top of STM image of H-8 shown in Figure 2D.
[1] D. Lehnherr, C. Chen, Z. Pedramrazi, C. R. DeBlase, J. M. Alzola, I. Keresztes, E. B. Lobkovsky, M. F. Crommie,
W. R. Dichtel, Chem. Sci. 2016, 7, 6357—6364. [PDF].